In a general sense, this term means the use of hydrogen as an energy carrier, i.e. as a medium for energy manipulation. In that sense, hydrogen has significant advantages over electricity, which is the most widespread energy carrier today. More precisely, hydrogen energy is the chemical energy stored in hydrogen.
Hydrogen economy is an integrated system of the global economy based on hydrogen as an energy carrier, which includes the production, transport, distribution and use of hydrogen energy.
Today’s economy is mainly based on electricity as a carrier, so it could be called the ‘electric economy’.
This is an attempt to highlight the idea of using hydrogen energy and its potential in meeting the energy needs of societies in the future.
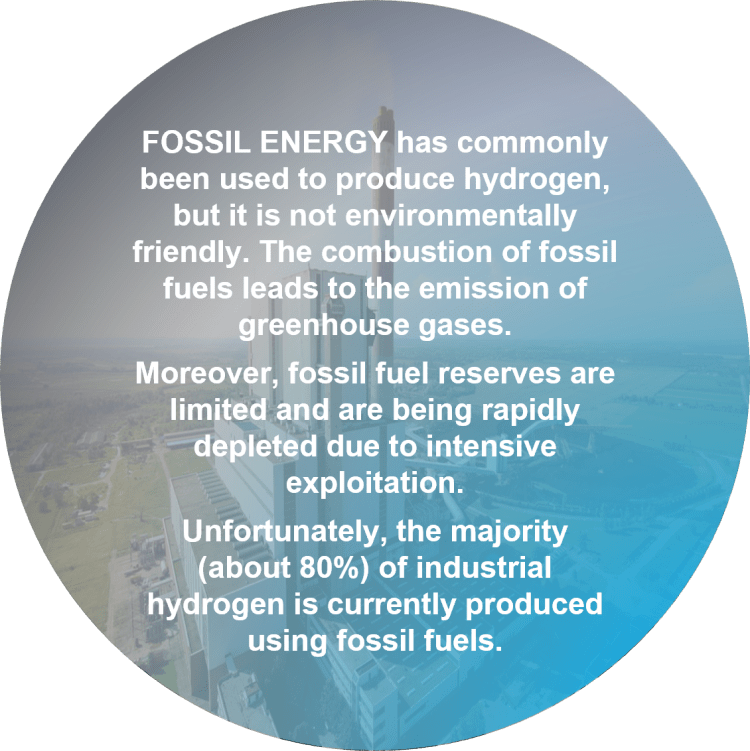
Hydrogen energy is not yet used on a large scale. It is a concept that is thought to reach its full significance after the depletion (or suspension) of fossil fuels.
HYDROGEN
- The lightest element in nature – Odorless and tasteless gas – It is both a fuel and a chemical – Flammable and explosive.
- Hydrogen is an element that has high chemical reactivity. It reacts with most elements making different compounds. Because of that, there is no free hydrogen in nature. It is found in the form of its compounds, the most important of which is water.
- Thus, it is not an energy raw material, but an energy carrier that must be produced from other materials using energy.
- The most important raw material for its production is water, although other natural materials can also be used, e.g. hydrocarbon fuels. However, the amount of water on Earth is unlimited, it is cheap, and the appropriate production methods are relatively simple, although they are energy demanding. Water is a “hard” molecule and a large amount of energy is required to break it down.
- In this way, part of the spent energy is stored in hydrogen, so that it is also a medium for energy storage.
- Hydrogen is an environmentally friendly fuel (provided it is produced using clean energy), whether it is used to generate heat or electricity. Besides ENERGY, the only product it generates is WATER: H2 + ½O2 → H2O.

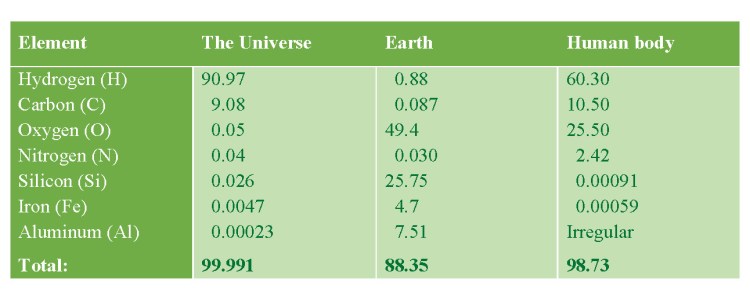
ABUNDANCE OF SOME CHEMICAL ELEMENTS (wt%)
Hydrogen is the most abundant element in the Universe, but not on Earth!
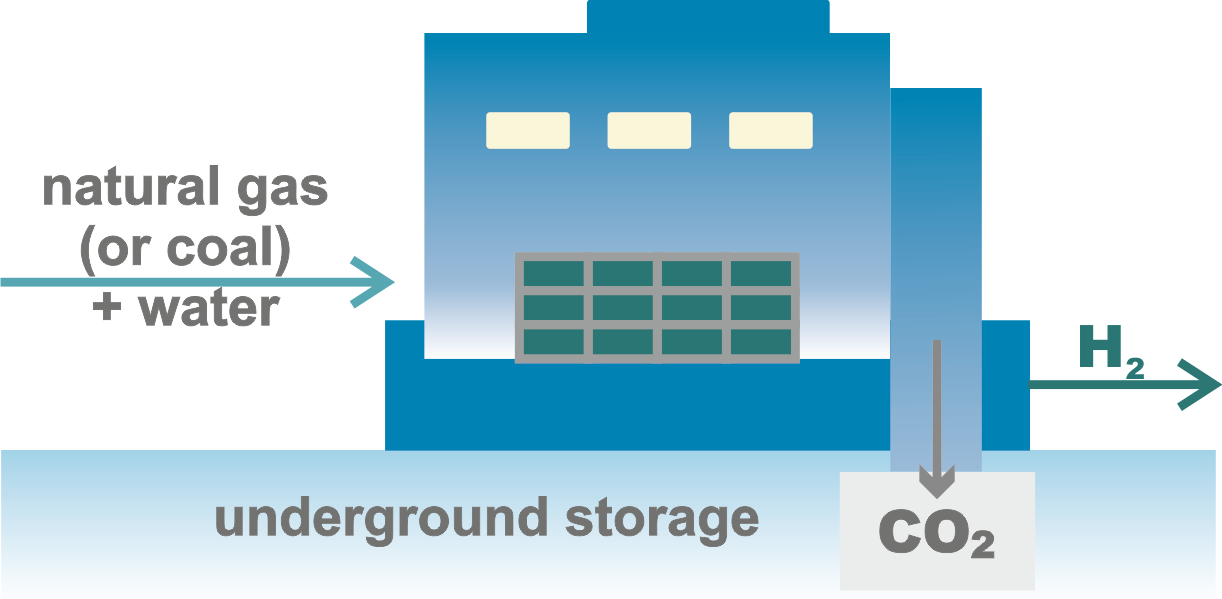
HYDROGEN PRODUCTION
NATURAL SOURCES OF GASEOUS HYDROGEN ARE NEGLIGIBLE.
The type of energy used and the production processes determine the type of hydrogen obtained. We can speak of at least three types of hydrogen:
GREY, BLUE, and GREEN.




Overview of hydrogen production methods according to the types of primary energy used.
Water, like any other compound, can be directly decomposed into hydrogen and oxygen at sufficiently high temperatures. However, since it is a very stable molecule, its complete dissociation would require extremely high temperatures. For illustration, even at 3000 °C the degree of water vapor decomposition would be only about 35%, and achieving and maintaining such a temperature under industrial conditions is difficult.
Therefore, various methods are being developed to decompose water at much lower temperatures, up to about 1000 °C. These include chemical decomposition (CD) based on fossil fuels, low-temperature processes—among which low-temperature water electrolysis (LTWE) is particularly important—as well as several high-temperature technologies still under development, such as high-temperature water electrolysis (HTWE), and thermochemical water-splitting cycles (TcWSC). These last two technologies are considered highly promising for future large-scale industrial production, for which the necessary high-temperature heat can be supplied either by specific nuclear reactors or by solar concentrators.
Brief descriptions of the main technologies used for water splitting can be accessed by clicking the links provided on the right.
There are several ways to produce hydrogen by splitting water using fossil fuels, but the technologies based on chemical decomposition are of the greatest industrial importance.
It should be noted that these methods yield grey hydrogen, priced at 1–2.1 $/kg, and blue hydrogen, priced at 1.5–2.9 $/kg.
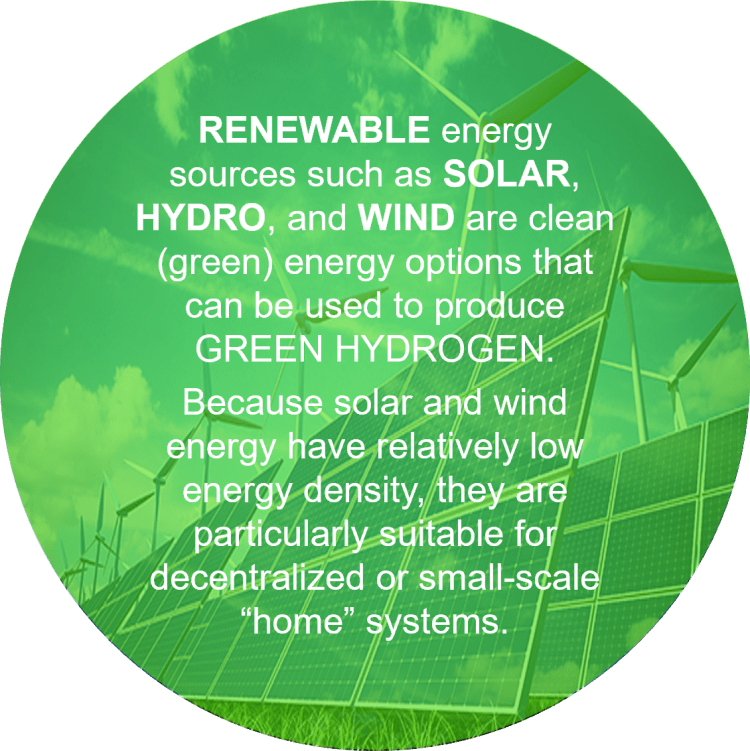
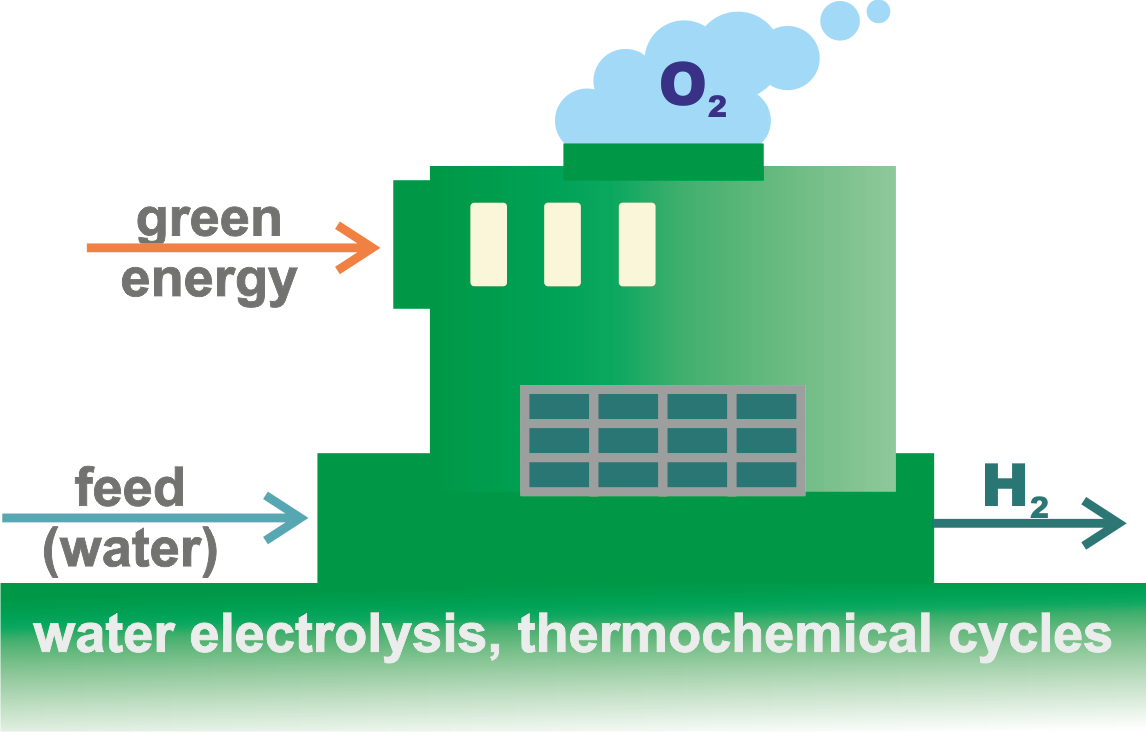
The distinctive feature of these renewable energy sources is that they generate electricity directly, which enables the production of (green) hydrogen. This primarily refers to electrolytic water splitting—a well-established electrochemical technology capable of producing high-purity hydrogen (up to 99.9%) in a single processing step—for which two main approaches exist: LTWE and HTVE (see bellow). The electrical energy used for electrolysis may, of course, also come from fossil-fuel power plants, where CO₂ emissions are unavoidable, as well as from nuclear power plants, which do not produce such emissions.
Green hydrogen is relatively expensive, with a current price of about 3.6–5.8 $/kg, although this cost is expected to fall significantly in the future as technology advances.

In addition, these reactors can be engineered to deliver very high temperatures. Such systems—particularly Very High Temperature Reactors (VHTR)—are exceptionally well suited for producing green hydrogen using high-temperature technologies, such as HTWE and TcWSC. For this reason, these technologies are important for meeting the goals of the global Nuclear Hydrogen Initiative (NHI).

HYDROGEN is both a Fuel and a Chemical!
Therefore, its application is possible in a number of areas:
- for direct heat generation,
- for direct production of electrical energy in fuel cells,
- Due to the great advantages that fuel cells have over other ways of producing electricity from hydrogen, they play a key role in hydrogen energy concepts,
- as an industrial chemical or raw material.
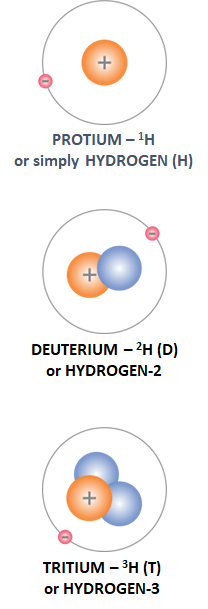
Abundance in nature: 99.985 %.
Main raw materials for production: WATER.
Production methods: There are several methods, but the most promising for large scale production in the future will be high-temperature electrolysis and thermochemical cycles.
Uses: FUEL, ENERGY CARRIER, chemical …
Abundance in nature: 0.015 %, or one D-atom per 6,500 hydrogen atoms.
Raw materials for production: WATER.
Production methods: chemical exchange, distillation, electrolysis and their combinations.
Uses: NEUTRON MODERATOR IN NUCLEAR FISSION (heavy water – D2O) REACTORS, POSSIBLY A FUEL COMPONENT IN FUSION THERMONUCLEAR REACTORS, TOGETHER WITH TRITIUM.
Abundance in nature: It is a radioactive isotope of cosmogenic origin, which has a half-life of 12.3 years. Accordingly, its concentration is T : H = 1 : 1018. Thus, the total amount of T on Earth is only 3.5 kg.
Production method: From lithium (Li) isotopes via nuclear reactions.
Uses: As a tracer in biochemical processes, as a source of light for safety signs, for monitoring of groundwater flows and, in the future, as a FUEL COMPONENT IN FUSION THERMONUCLEAR REACTORS.
Non-Energy Uses – HYDROGEN as a Raw Material
- Ammonia Synthesis
- Synthesis of Methanol
- Direct Reduction of Iron Ore
- Refinery Hydrogenation
- Coal Gasification
- Nickel Manufacturing
- Glass Manufacturing
- Pharmaceutical Industry
- Semiconductor Manufacturing
- Generator Cooling
- Argon Purification
- Meteorology

Hydrogen Fuel Cell
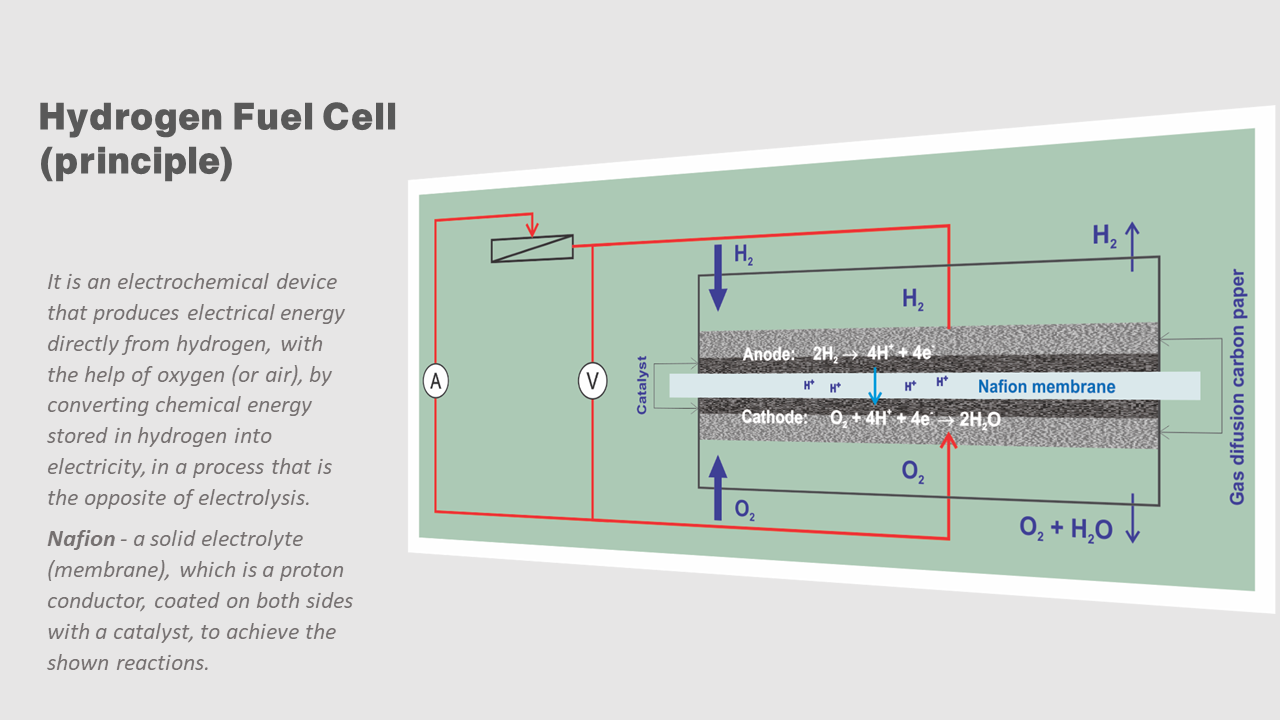
IN FACT, THE FULL EFFICIENCY OF THE USE OF HYDROGEN FUEL CELLS IS ACHIEVED THROUGH ADEQUATE ENERGY CONCEPTS. THE TYPE OF CONCEPT DEPENDS ON THE NEEDS AND POSSIBILITIES OF USING HYDROGEN ENERGY.
The picture below shows one of a number of possible concepts. It is quite specific because, in addition to being one of the ways to manipulate hydrogen energy, it also takes into account hydrogen isotope effects.
Energy Storage using Hydrogen – The Principle of a Reversible Power Plant
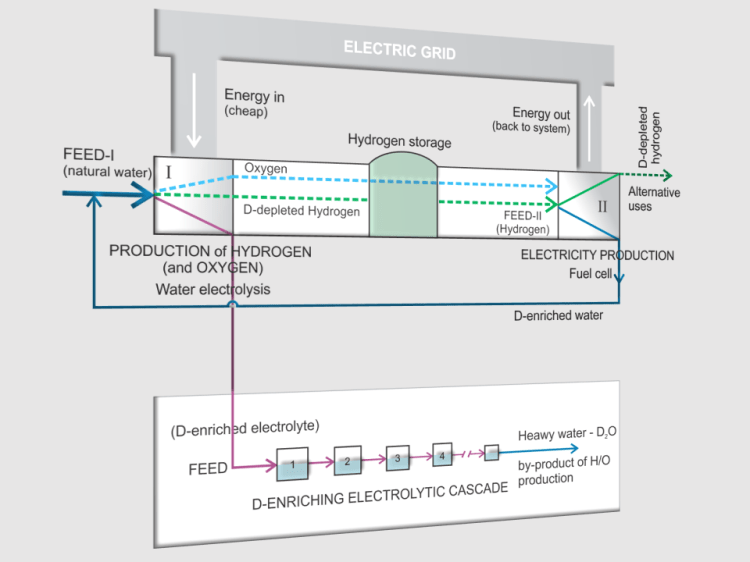
- This concept is a combination of energy manipulation in the (electrical) energy system with the help of hydrogen as a medium, for its STORAGE in periods of reduced consumption, and with the use of water electrolysis and fuel cells.
- In periods of increased demand, the energy stored in hydrogen is converted into electricity in fuel cells and returned to the system.
- In this way, the CYCLING OF ENERGY takes place. The idea is based on the principle of REVERSIBLE POWER PLANT.
- Of course, due to unavoidable losses during cycling, the amount of output energy is less than the amount of input. These losses, together with the costs of transport and storage, are the dominant contributors to the cost of storage.
- The transformation of one compound into another leads to the so-called ISOTOPE EFFECT, i.e. to a change in the ratio of isotope concentrations.
- Here, the electrolyzer and fuel cell play the roles of ISOTOPE SEPARATION UNITS.
- Thus, electrolysis converts water (as an input stream) into hydrogen (and oxygen), whereby the outgoing hydrogen, as a less dense phase, is depleted in the deuterium isotope (D), while the water remaining in the electrolyte is enriched. A similar thing happens during the transformation of hydrogen into water in the fuel cell.
- By multiple electrolysis of such an electrolyte, pure HEAVY WATER (D2O) can be obtained as a by-product, which reduces energy storage costs.

A futuristic view of hydrogen energy
As mentioned earlier, hydrogen can be produced using any type of primary energy. Once generated, it is stored temporarily, serving as a buffer between production and consumption and contributing to overall system stability. When energy is needed, hydrogen can be transported through gas pipelines to urban areas, where it may be used to produce electricity and heat or serve as fuel for various transport systems. In smaller or decentralized systems, hydrogen can also be delivered by trucks, trains, ships, and other transport modes.



