Coal is the most widely and evenly distributed energy resource, available in sufficient quantities in more than one hundred countries. This enables many nations to produce their own electricity—often a key factor for maintaining sovereignty. In some countries, coal is the only reliable energy source. For less developed or economically weaker states, replacing fossil fuels—particularly coal—within a short period is extremely challenging. Therefore, it is essential to find ways to extend the transition period while, at the same time, developing non-carbon energy sources. The project outlined here offers one such idea, without going into technological details.
PROJECT 1
The combustion of coal primarily releases carbon dioxide and heat (ΔH). Thus,
1. LIGNITE (of medium quality) + O₂ → CO₂ + … ΔH ≈ −120 kJ/mol
When the generated heat is used in a thermal power plant, about Eel ≈ 40 kJ/mol of electrical energy is obtained.
The emitted CO₂ can be converted into methane with the help of hydrogen. This is the so-called methanation of carbon dioxide, which eliminates CO₂ from emissions while simultaneously storing the energy used to produce the hydrogen. The process is based on the Sabatier[1] reaction, which has a molar ratio of 1:4:
2. CO2 + 4H2 → CH4 + 2H2O ΔH = -165 kJ
The mechanism is complex and involves the use of a catalyst. It takes place at temperatures between 250 °C and 400 °C and under elevated pressures. The reaction releases enough heat to be self-sustaining. Only a certain amount of energy is required to initiate it.
Methanation uses green hydrogen, in which green energy is stored. Such hydrogen can be produced using energy from renewable sources or from advanced nuclear systems. Therefore:
3. 4H2O → 4H2 + 2O2 ΔH = +1144 kJ
Through methanation of CO2, part of the hydrogen’s energy is “re-stored” in methane. Thus, methane becomes a unique storage medium for:
- hydrogen,
- energy, and
- carbon dioxide.
Furthermore, combustion of methane releases carbon dioxide, water, and a significant amount of energy:
4. CH4 + 2O2 → CO2 + 2H2O ΔH = −891 kJ/mol
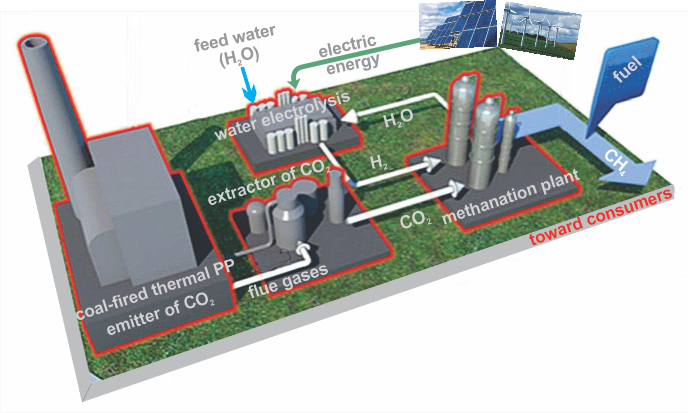
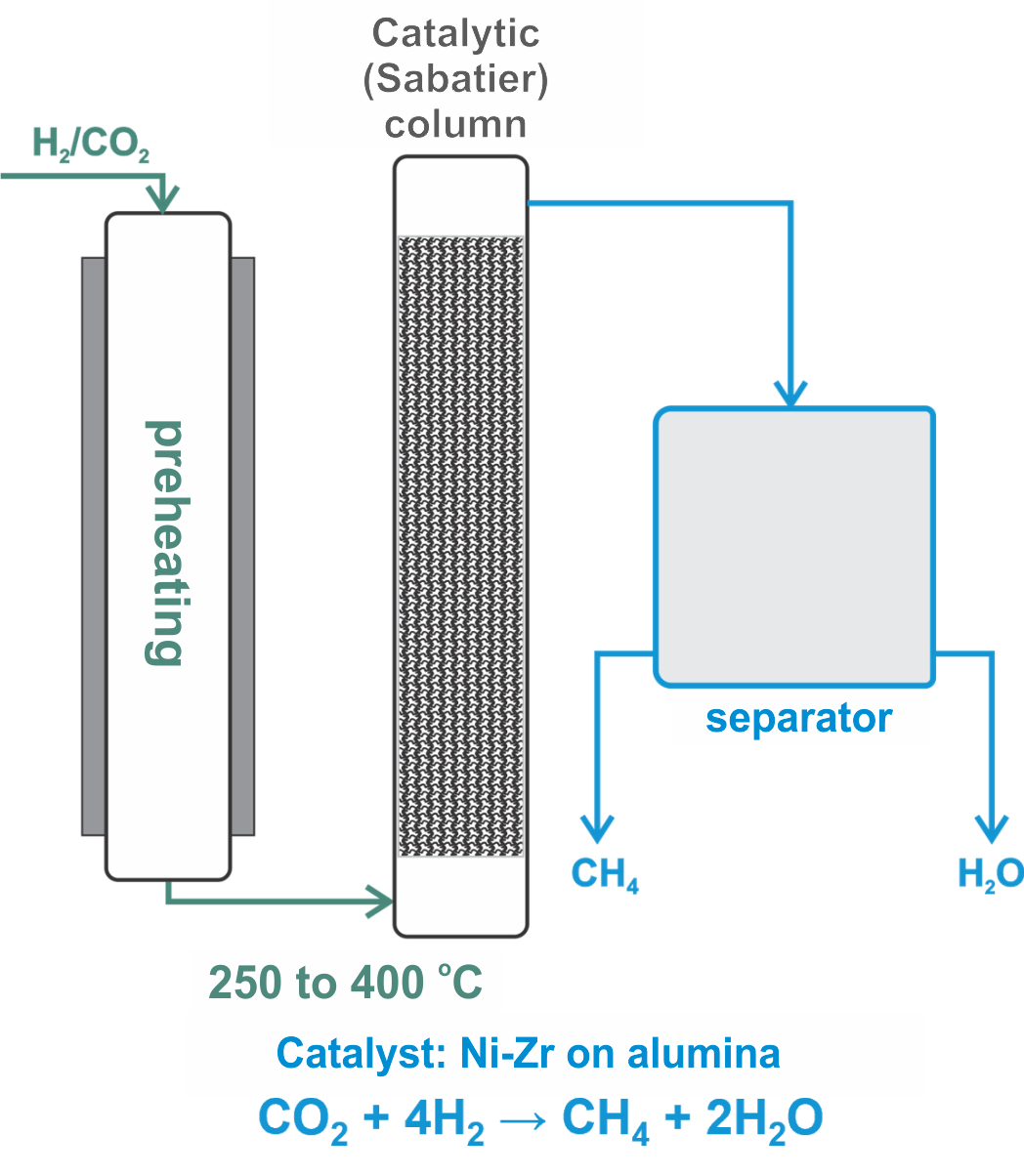
Schematic of a CO₂ methanation plant and the methane production unit within it.
[1] Paul Sabatier, a French chemist and Nobel Prize winner in Chemistry in 1912.

CO2
THE ABOVE FACTS
offer the possibility of using CO2 methanation to slow down COAL PHASE-OUT on the path toward ENERGY TRANSITION.

PRACTICAL MEANING
…
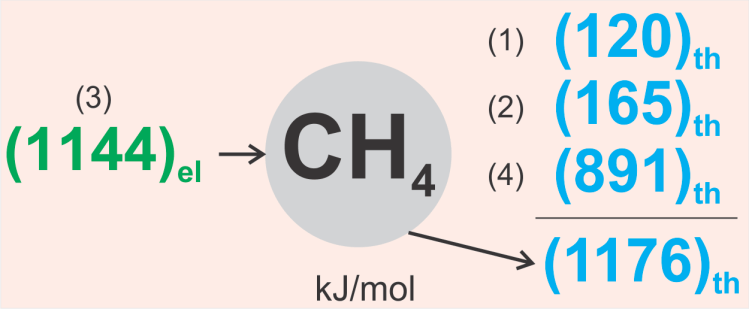
Finally, the energy balance of the entire system, based on the chemical equations presented, is shown in the figure. At first glance, it appears that the amount of energy required to produce hydrogen and the amount of energy returned are formally the same. However, this is not the full picture.
To make these energies comparable, the amount of electrical energy consumed must be multiplied by a conversion factor—typically at least 2 or more—to obtain the corresponding amount of primary energy.
This means that the energy balance of methanation is negative, which raises THE QUESTION of whether it is a reasonable approach, despite its negative energy efficiency.
THE ANSWER
In principle, the answer is YES — but only under certain conditions. Methanation is not intended to be a process that yields more energy than it consumes. Instead, it is a power-to-gas conversion technology, whose value does not lie in economic benefits, but first and foremost in environmental protection, energy storage, and system stability.
- A crucial requirement for the entire system is that the electrical energy used to generate hydrogen must be inexpensive. This is usually achieved by using excess electricity available during periods of low demand. In this way, the need for energy storage is aligned with the need to eliminate CO₂ emissions. Instead of curtailing power production when there is surplus electricity, that surplus is used to produce hydrogen and methane.
- Methanation is particularly advantageous in energy systems dominated by coal-fired power plants, especially when non-carbon energy sources for hydrogen production are simultaneously being deployed and expanded.
- An additional advantage is the reduction of CO₂-related taxes, which are increasing rapidly.
- The advantage also lies in the fact that methane delivers significantly more energy per unit mass than coal, especially lignite. This additional energy comes from the hydrogen used for methanation, which indirectly creates conditions for reducing the production of “coal” energy. The development of green energy through capacity expansion automatically means a reduction in coal-based production. Methanation can therefore serve as a transitional solution during the ENERGY TRANSITION, until sufficient green capacity is built to eliminate coal completely.
- The existing infrastructure for handling natural gas (methane) is well developed worldwide, including its use in automobiles with conventional gasoline engines, further supports this approach. Unlike hydrogen, methane can be stored in the current gas infrastructure, in underground storage facilities, or transported through established gas pipelines. These pipelines can carry methane over thousands of kilometers with relatively low losses.
- Methane can serve as an energy buffer for strategic security of supply, maintaining stability during periods when energy production, especially renewable, is insufficient.
END OF THE PROJECT 1 SUMMARY
