
Nuclear energy in the broadest sense originates from the transmutations of the atomic nuclei of a chemical element into the nuclei of other elements, either through the processes of radioactive decay or nuclear reactions.
In fact, nuclear energy is the energy that comes from the fission of atomic nuclei, allowing us to produce energy at an industrial scale.
At the same time, nuclear fusion has an energy potential even higher than that of fission, but it has not yet been brought to commercial exploitation, despite enormous research and technical efforts.
One of the most important nuclear terms is related to the concept of isotopes.
Isotopes are atoms of the same element that have different masses due to the different number of neutrons in their nuclei. Their behavior in many processes shows small but visible differences. This fact enables the separation of isotopes, i.e. concentrating one isotope in a mixture by removing others.
It is of crucial importance for the production of essential materials for nuclear energy.
Useful Links
FISSION
FACTS ABOUT NUCLEAR FISSION
- In the usual sense, nuclear fission is a type of nuclear reaction in which a heavy atomic nucleus, when hit by a neutron, splits into two lighter nuclei (fission products). Different nuclei of an isotope can be split in different ways. All resulting nuclei are radioactive.
- A huge amount of energy is released – about 200 MeV per fission in the case of uranium fission. That means that the energy density is EDFIS ≈ 79 400 000 МЈ/kg U.
- On average, up to three neutrons are released per fission.
- Not every atomic nucleus will undergo fission with high efficiency, but only the nuclei of certain isotopes of some chemical elements, called fissile isotopes.
- Those isotopes are uranium-235 (naturally occurring – 0.7% in natural U), uranium-233 (artificial, made from thorium-232), and plutonium-239 (artificial, made from uranium-238 – 99.3% in natural U).
- The production of artificial fissile isotopes from their naturally occurring non-fissile parents is usually achieved through the conversion of fuel using so-called breeder reactors. These reactors can create more fissile material than they consume.
FUSION
FACTS ABOUT THERMONUCLEAR FUSION
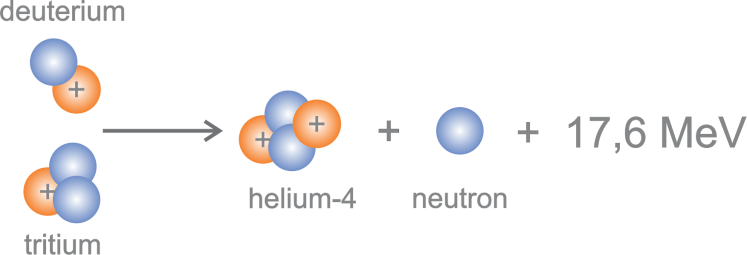
- The only fusion of all investigated to date, which has the potential to become a commercial method for energy production, is D-T fusion, because it has the highest (although still low) efficiency, the lowest temperature threshold (about 100 million degrees Celsius), releases a relatively large amount of energy (17.6 MeV per fusion – EDFUZ ≈ 679 000 000 МЈ/kg D-T), raw materials for fuel production are available.
- Since extremely high temperatures are necessary for fusion to occur, it is commonly called thermonuclear fusion.
- Although it is a seemingly simple reaction, its use is quite far from industrial level (at least several tens of years), despite the great scientific, technical, and financial efforts invested in its development.
- Fuel for the fusion reactor is a Deuterium-Tritium (1:1) mixture. DEUTERIUM (D) is a naturally occurring, stable, heavy isotope of hydrogen. Its concentration in nature is about 0.015 %. The raw material for its production is water. TRITIUM (T) is a super-heavy, radioactive isotope of hydrogen. Total amount of T on Earth is about 3.5 kg, thus it must be produced, and it is produced by neutron reactions from LITHIUM.
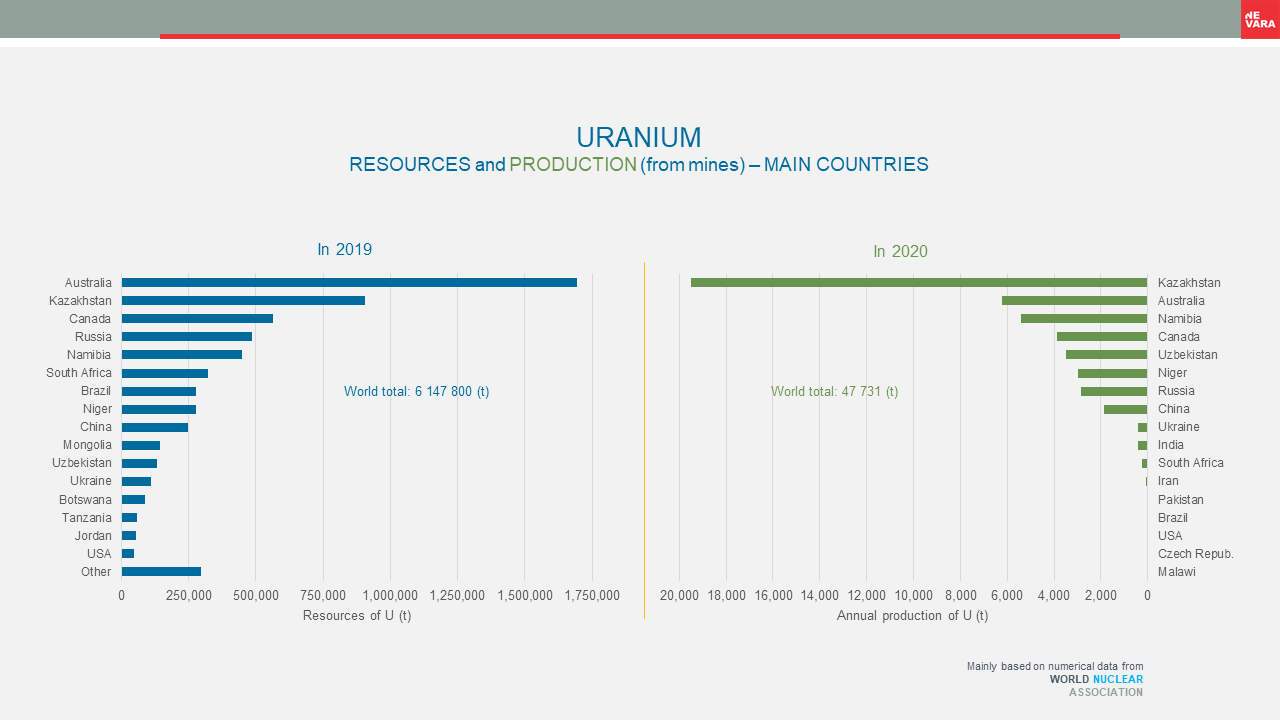
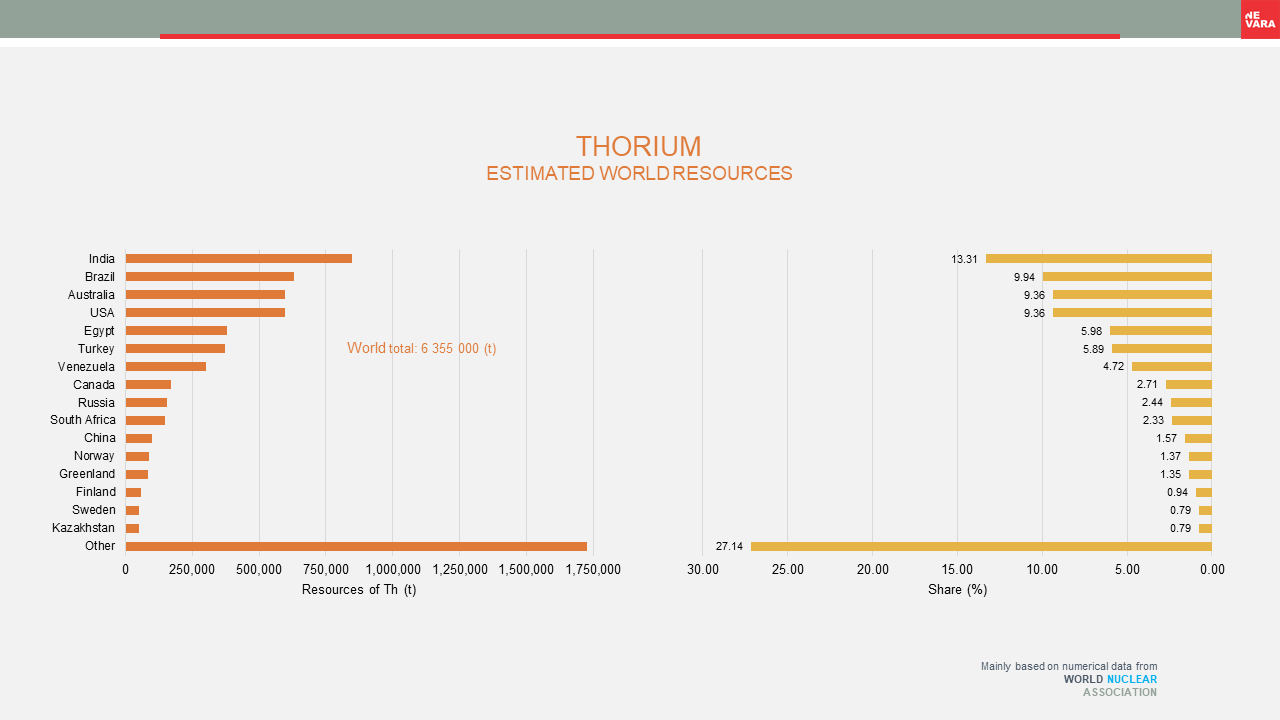
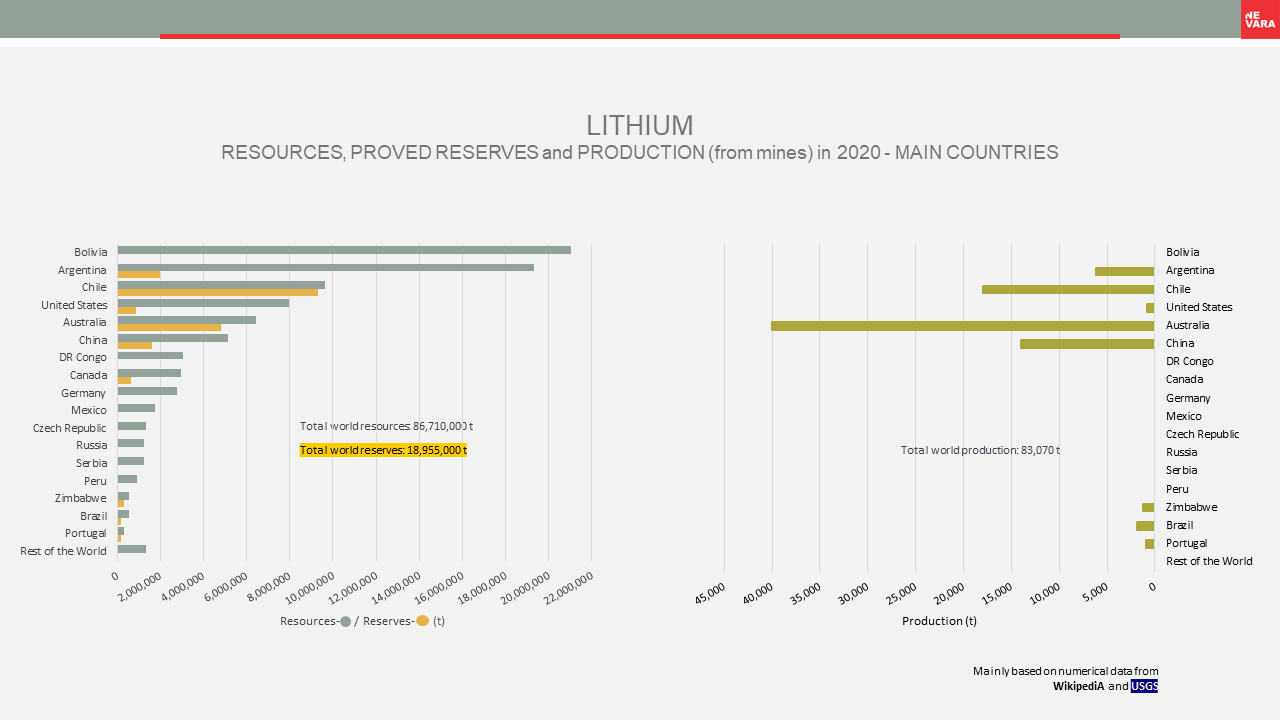
Nuclear raw materials




One thought on “NUCLEAR ENERGY”